15+ Calculate Zeff For A Valence Electron In An Oxygen Atom
Calculate Zeff for the following. Ms and 5191023 kg.
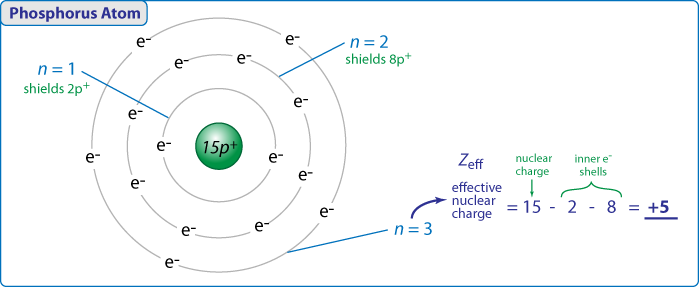
Effective Nuclear Png
The Magnesium Has 2 Valence Electron While The Oxygen Misses 2 Electron To Complete Its Octet.
. The electron from a hydrogen atom drops from an excited state into the ground state. Calculate the for the valence electron in an oxygen atom. Calculato Zeff for a valence electron in an oxygen atom.
Express your answer numerically. Like seriously you posted that answer 7years. Who are you and how did you know that.
Use the periodic table to determine the number of protons in a copper atom. Who are you and how did you know that. The effective nuclear charge experienced by a valence electron on an O.
Calculate zeff for a valence electron in an oxygen. Express your answer numerically. You have been asked.
An atom is always more stable when it has 8 valence electrons. To determine the value for S start by writing out the electron configuration for the atom. So shielding S 035 x 5 085 x 2 345.
View Available Hints vo ΑΣφ Zer_eff 6 Submit Previous Answers X Incorrect. Therfore shielding S 035 x 5 085 x 2 345. I just need help on 3 6 and 7 Please explain answers 2.
Calculate zeff for a valence electron in an oxygen atom. O 1s2 2s 2p6. A A valence electron in an oxygen atom b A valence electron in an iron atom c A 2p electron in an iron atom 7.
Zeff Z S. The effective nuclear charge experienced by a valence electron on an O atom is 455. View Available Hint s IVO ΟΙ ΑΣΦ.
1What is the magnitude of. Calculate the for the valence electron in an oxygen. Express your answer numerically.
The electron and neutrino are emitted at right angles and have momenta of 9151023 kg. Up to 256 cash back Get the detailed answer. When an electron drops into a lower-energy orbital energy is released in the form of electromagnetic.
Zeff Z S 8 345 455. 8 345. Which of the following compounds contains oxygen has no absorption above 3100 cm1 and none between 1650 and 1780 cm1.
Calculate zeff for a valence electron in an oxygen atom. Chemistry questions and answers. Jpdvolleybal1326 jpdvolleybal1326 11172017 Chemistry College.
Which would you expect to experience a greater effective nuclear charge a 2p electron of a Ne atom or a 3s electron of. Zeff Z S 8 345 455. Part A Calculate Zeff for a valence electron in an oxygen atom.
The effective nuclear charge experienced by a valence electron in an O atom is 455. Up to 256 cash back Get the detailed answer. The effective nuclear charge experienced by a valence electron in an O atom is 455.

Solved Write The Complete Ground State Electron Chegg Com
How To Find The Effective Nuclear Charge Of Oxygen Quora

Calculate The Effective Nuclear Charge Of The Last Electron In An Atom The Electronic Configuration Is 1s 2 2s 2 2p 6 3s 2 3p 5
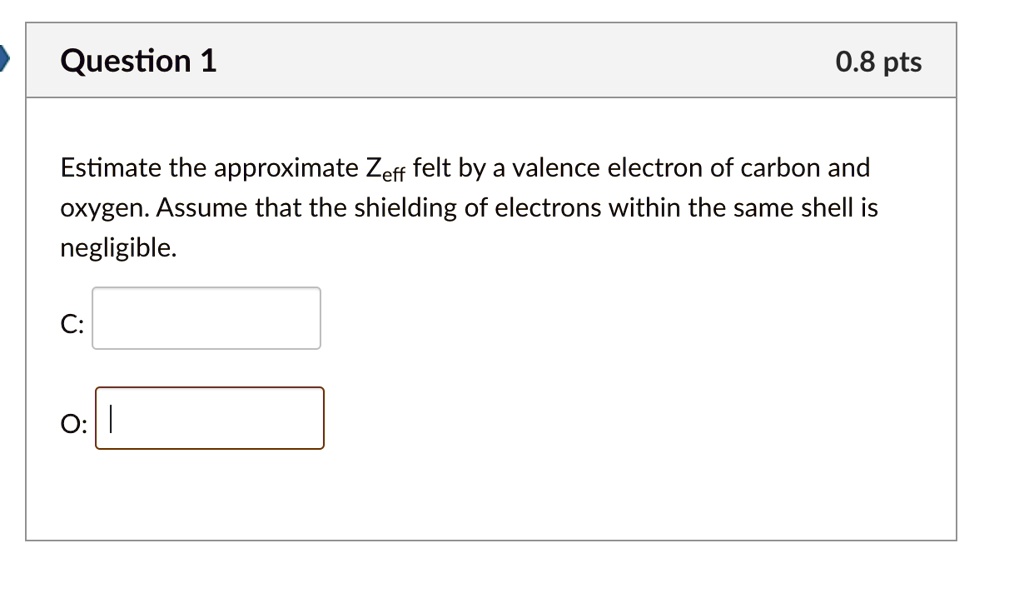
Solved Question 1 0 8 Pts Estimate The Approximate Zeff Felt By A Valence Electron Of Carbon And Oxygen Assume That The Shielding Of Electrons Within The Same Shell Is Negligible

Calculate The Effective Nuclear Charge For Oxygen Homework Study Com
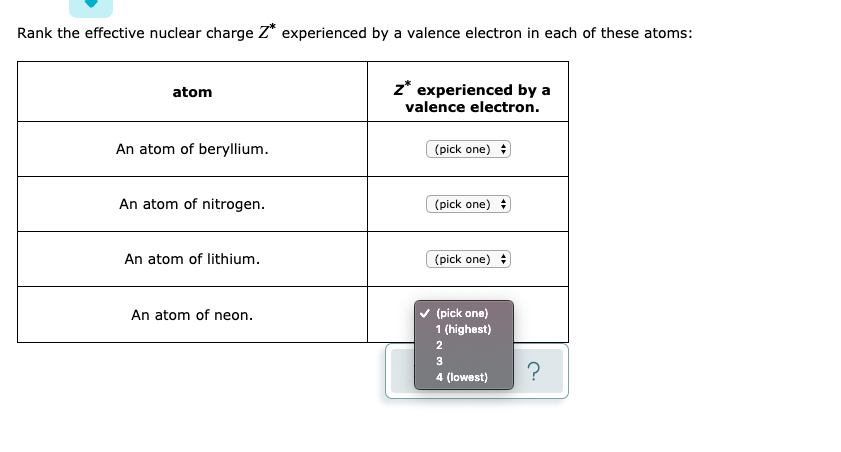
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com

How To Find The Effective Nuclear Charge Of Oxygen Quora

Calculate The Effective Nuclear Charge At The Periphery Of Nitrogen Atom When An Extra Electron Is Added In The Formation Of Anion Also Calculate The Effective Nuclear Change Of N Atom

The Diagram Shows The Electron Configuration Around The Chlorine Nucleus Chlorine Has 17 Brainly Com

How To Find The Effective Nuclear Charge Of Oxygen Quora

Calculate The Effective Nuclear Charge At The Periphery Of Nitrogen Atom When An Extre Electron Is Added In The Formation Of Anion Also Calculate The Effective Nuclear Charge Of N Atom

Solved Calculate The Effective Nuclear Charge On A Valence Electron In An Oxygen Atom

Pdf Slater S Rules Kajal Panda Academia Edu
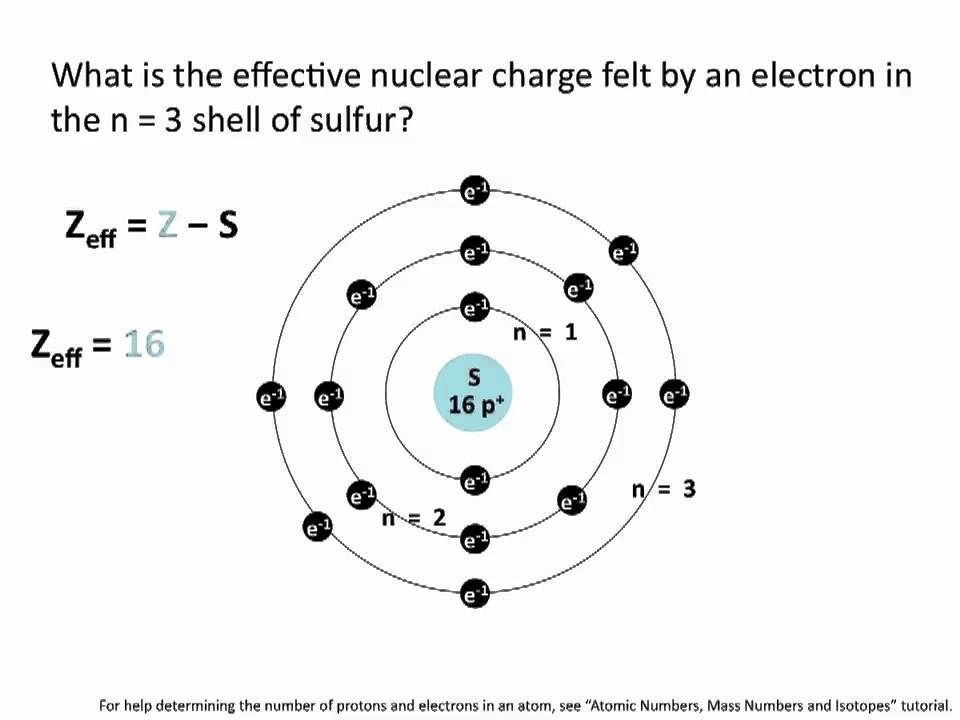
Effective Nuclear Charge Chemistry Tutorial Youtube
Ptable Color Svg

Solved Effective Nuclear Charge And Ionization Energy Chegg Com
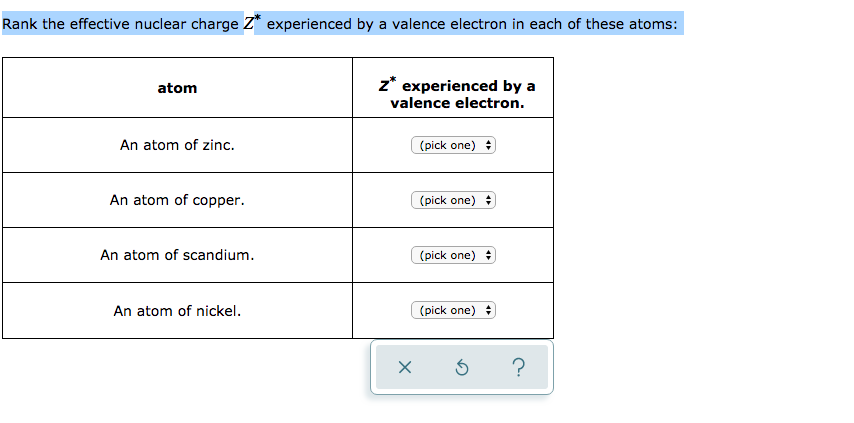
Answered Rank The Effective Nuclear Charge Z Bartleby